-
Services
-
Practice Areas
- Banking and Finance
- Capital Markets
- Corporate M&A
- Dispute Resolution
- Employment and Labour
- EU and Competition Law
- Healthcare, Life Sciences & Pharmaceuticals
- Intellectual Property
- Planning and Land Use
- Projects and Energy
- Public Law
- Real Estate and Tourism
- Responsible Business
- Tax
- Technology, Media and Telecommunications
-
Sectors
- Agribusiness
- Banking and financial institutions
- Distribution and retail
- Education
- Energy and natural resources
- Government and public sector
- Healthcare, life sciences and pharmaceuticals
- Infrastructure
- Insurance and pension funds
- Manufacturing
- Mobility, transport and logistics
- Planning and land use
- Real estate and construction
- Social economy
- Sports
- Tourism and leisure
- Desks
- Buzz Legal
-
Practice Areas
-
People
-
Knowledge
-
Newsletter SubscriptionKeep up to date
Subscribe to PLMJ’s newsletters to receive the most up-to-date legal insights and our invitations to exclusive events.
-
-
About Us
-
Apply hereWe invest in talent
We are looking for people who aim to go further and face the future with confidence.
-
- ESG
-
Services
-
Practice Areas
- Banking and Finance
- Capital Markets
- Corporate M&A
- Dispute Resolution
- Employment and Labour
- EU and Competition Law
- Healthcare, Life Sciences & Pharmaceuticals
- Intellectual Property
- Planning and Land Use
- Projects and Energy
- Public Law
- Real Estate and Tourism
- Responsible Business
- Tax
- Technology, Media and Telecommunications
-
Sectors
- Agribusiness
- Banking and financial institutions
- Distribution and retail
- Education
- Energy and natural resources
- Government and public sector
- Healthcare, life sciences and pharmaceuticals
- Infrastructure
- Insurance and pension funds
- Manufacturing
- Mobility, transport and logistics
- Planning and land use
- Real estate and construction
- Social economy
- Sports
- Tourism and leisure
- Desks
- Buzz Legal
-
Practice Areas
-
People
-
Knowledge
-
Newsletter SubscriptionKeep up to date
Subscribe to PLMJ’s newsletters to receive the most up-to-date legal insights and our invitations to exclusive events.
-
-
About Us
-
Apply hereWe invest in talent
We are looking for people who aim to go further and face the future with confidence.
-
- ESG
Informative Note
Authorisation and review of medicine prices
19/11/2025Criteria to be in force in 2026
Ministerial Order 394/2025/1 of 14 November was published in order to define the reference countries to be used in 2026 and the exceptional criteria to be applied in the review of the prices of medicines.
Reference countries for new prices and for the annual price review
The reference countries for the approval of new prices and the annual price review of medicines (outpatient and hospital market) will continue to be Spain, France, Italy, and Belgium.
Criteria to be observed in the Annual Review of Medicine Prices (APR)
Outpatient market (non-generic and non-biosimilar medicines)
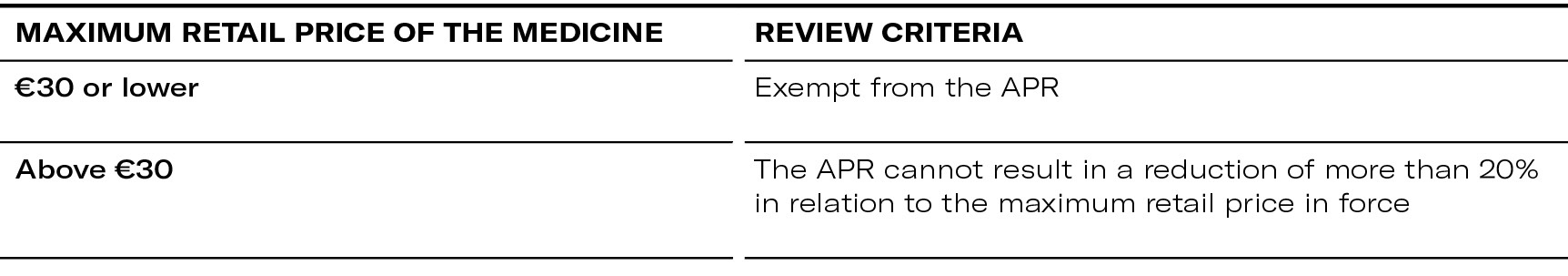
Hospital market (non-generic and non-biosimilar)

Outpatient and hospital market (generic and biosimilar medicines)
All generic and biosimilar medicines are exempt from the APR, except for generic medicines with a maximum price higher than the maximum price of the reference medicine resulting from the 2026 APR. In this case, the maximum price of the generic medicine cannot exceed the maximum price of the reference medicine.
Critical medicines
Medicines included on the list of essential critical medicines, which are dispensed in the outpatient market, are exempt from APR.
Deadlines for marketing authorisation holders or their legal representatives to submit the prices to be applied in 2025:
- Annual review of the maximum retail price of non-generic medicines: By 15 December 2025, with prices taking effect on 1 January 2026
- Annual review of maximum retail price for generic and biosimilar medicines: By 15 January 2026, with prices taking effect on 1 February 2026